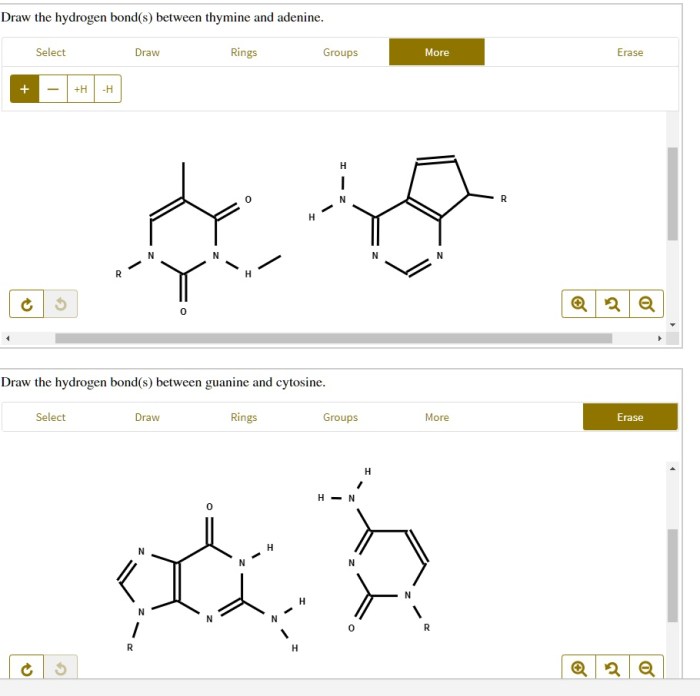
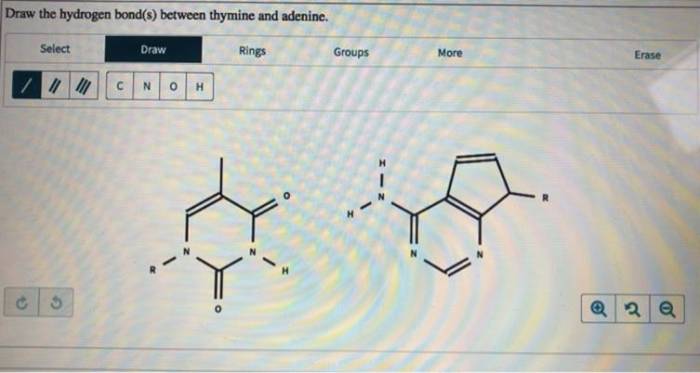
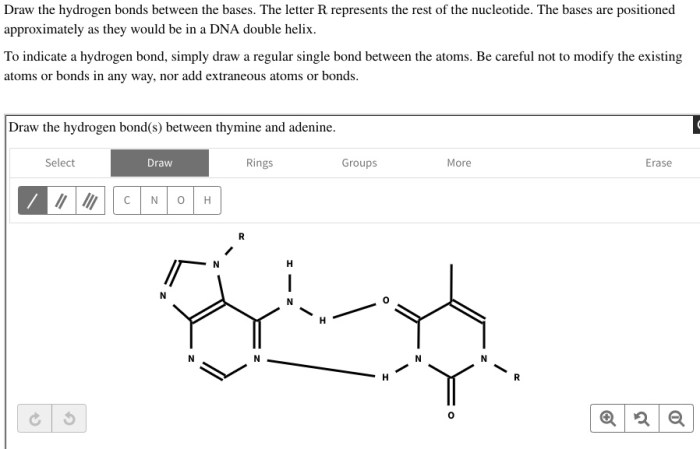
Draw the hydrogen bonds between thymine and adenine – In the realm of genetics, the hydrogen bonds between thymine and adenine play a pivotal role in maintaining the structural integrity and functionality of DNA. This guide delves into the intricacies of these crucial interactions, exploring their significance, experimental techniques used to study them, and their implications for DNA structure and function.
Hydrogen bonding, a fundamental force in molecular biology, is responsible for the specific pairing of thymine with adenine in the DNA double helix. These bonds form between the electronegative nitrogen and oxygen atoms of the two bases, creating a stable and specific molecular arrangement.
Hydrogen Bonding between Thymine and Adenine: Draw The Hydrogen Bonds Between Thymine And Adenine
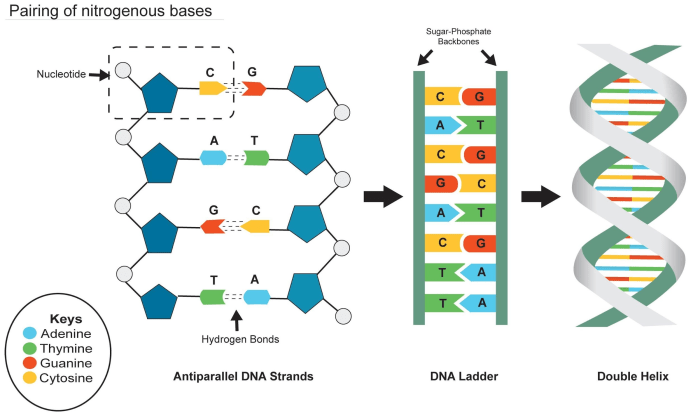
Hydrogen bonding is a critical force in maintaining the structure and function of DNA. It is a type of non-covalent bond that forms between electronegative atoms, such as nitrogen and oxygen, and hydrogen atoms. In DNA, hydrogen bonding occurs between the complementary base pairs adenine (A) and thymine (T).
Specifically, two hydrogen bonds form between thymine and adenine. One hydrogen bond forms between the amino group (NH 2) of adenine and the carbonyl group (C=O) of thymine. The second hydrogen bond forms between the imine group (NH) of thymine and the amino group (NH 2) of adenine.
These hydrogen bonds are essential for maintaining the double helix structure of DNA. They hold the two strands of DNA together, forming a stable and specific structure. The specificity of hydrogen bonding ensures that only complementary base pairs can pair with each other, maintaining the genetic code.
Significance of Hydrogen Bonding in DNA Structure
Hydrogen bonding plays a crucial role in maintaining the double helix structure of DNA. It contributes to the stability and specificity of DNA, enabling it to carry and transmit genetic information accurately.
- Stability:Hydrogen bonds provide stability to the DNA double helix by holding the two strands together. They prevent the strands from separating, ensuring the integrity of the genetic information.
- Specificity:Hydrogen bonding ensures that only complementary base pairs can pair with each other. This specificity is essential for maintaining the genetic code and preventing errors during DNA replication and transcription.
Hydrogen bonding also affects DNA replication and transcription. During replication, the hydrogen bonds between base pairs must break to allow the DNA strands to separate. During transcription, hydrogen bonding between the template strand and RNA nucleotides ensures the correct incorporation of nucleotides into the RNA molecule.
Comparison with Other DNA Base Pairs, Draw the hydrogen bonds between thymine and adenine
The hydrogen bonding pattern between thymine and adenine is unique compared to other base pairs in DNA.
- Guanine-Cytosine (G-C):G-C base pairs form three hydrogen bonds instead of two. This additional hydrogen bond makes G-C pairs more stable than A-T pairs.
- Inosine-Cytosine (I-C):I-C base pairs form only one hydrogen bond. This weak hydrogen bonding contributes to the flexibility and dynamic nature of certain DNA regions.
The differences in hydrogen bonding strength and specificity among different base pairs have implications for DNA structure and function. For example, the higher stability of G-C pairs contributes to the formation of stable regions in DNA, such as the centromeres and telomeres.
Experimental Techniques for Studying Hydrogen Bonding
Various experimental techniques are used to study hydrogen bonding in DNA, including:
- X-ray crystallography:This technique provides detailed structural information about DNA, including the positions and orientations of hydrogen bonds.
- NMR spectroscopy:This technique can detect and characterize hydrogen bonds based on their chemical shifts and relaxation times.
These techniques have been used to investigate hydrogen bonding in thymine-adenine pairs, providing insights into their structure and dynamics.
Quick FAQs
What is the significance of hydrogen bonding in DNA?
Hydrogen bonding is responsible for maintaining the double helix structure of DNA, providing stability and specificity to the genetic code.
How do hydrogen bonds contribute to DNA replication?
During DNA replication, hydrogen bonds between complementary base pairs facilitate the accurate pairing of nucleotides, ensuring the faithful transmission of genetic information.
What experimental techniques are used to study hydrogen bonding in DNA?
X-ray crystallography and NMR spectroscopy are commonly used to determine the structure and dynamics of hydrogen bonds in DNA.