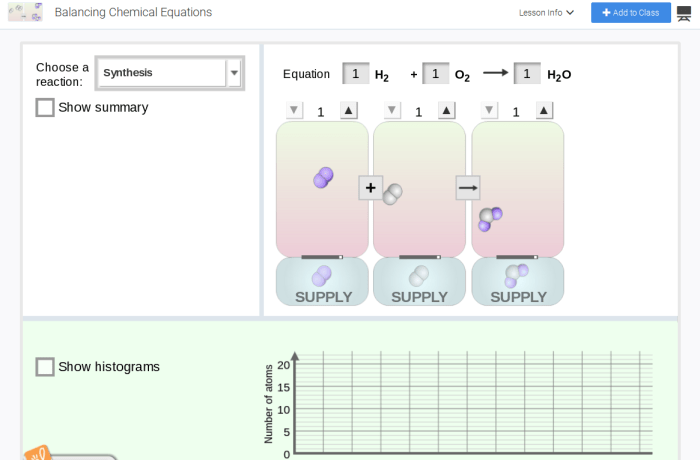
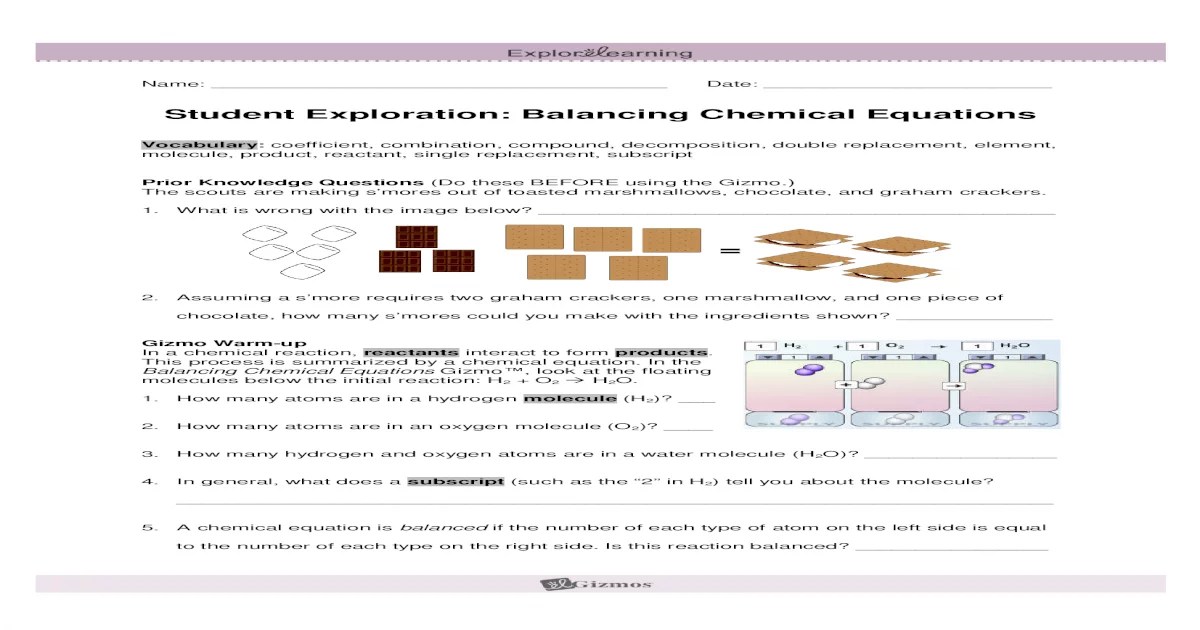
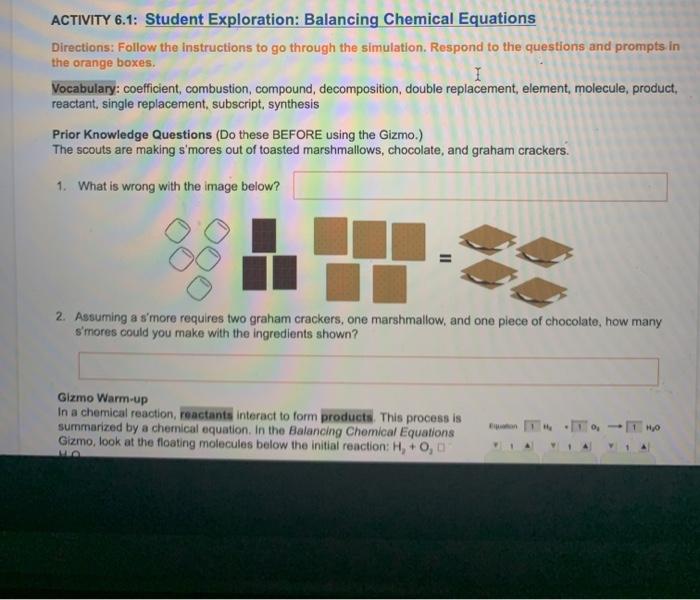
Embark on a captivating journey into the realm of chemical equation balancing with Student Exploration: Balancing Chemical Equations. This comprehensive guide unravels the intricacies of this fundamental concept, empowering students with the knowledge and skills to navigate the complexities of chemical reactions.
Delving into the core principles of equation balancing, this exploration unveils the significance of stoichiometry, introduces the concept of coefficients, and elucidates various balancing methods. Interactive simulations, engaging games, and hands-on experiments bring these concepts to life, fostering a dynamic and immersive learning experience.
Chemical Equation Balancing
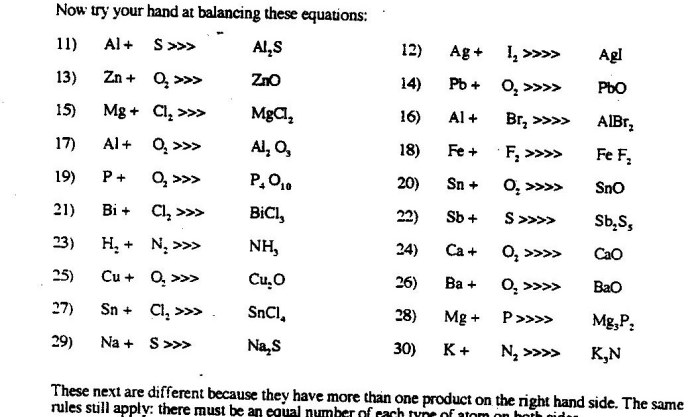
Balancing chemical equations involves adjusting the coefficients in front of each chemical formula to ensure that the number of atoms of each element is the same on both sides of the equation. This is essential for accurate representation of chemical reactions and for performing stoichiometric calculations.
For example, the unbalanced equation for the combustion of methane is:
CH4+ O 2→ CO 2+ H 2O
To balance this equation, we need to add coefficients to ensure that the number of atoms of each element is the same on both sides. The balanced equation is:
CH4+ 2O 2→ CO 2+ 2H 2O
Methods for Balancing Equations
There are several methods for balancing chemical equations, including:
- Trial and error: This method involves adjusting the coefficients until the equation is balanced.
- Half-reaction method: This method involves balancing the oxidation and reduction half-reactions separately.
- Oxidation-reduction method: This method is used for balancing redox reactions.
The choice of method depends on the complexity of the equation.
Applications of Balanced Equations
Balanced chemical equations are used for a variety of purposes, including:
- Predicting the products of a reaction
- Calculating the stoichiometry of a reaction
- Determining the limiting reactant
- Designing chemical processes
Challenges in Balancing Equations
Students often face challenges when balancing chemical equations. These challenges include:
- Identifying the type of reaction
- Counting atoms accurately
- Applying the correct balancing method
To overcome these challenges, students need to practice balancing equations regularly and seek help from teachers or tutors when needed.
Student Exploration Activities, Student exploration: balancing chemical equations
There are several engaging activities that can help students explore chemical equation balancing. These activities include:
- Interactive simulations
- Games
- Hands-on experiments
These activities can help students visualize the process of balancing equations and make learning more interactive.
Assessment and Evaluation
To assess student understanding of equation balancing, teachers can use a variety of assessment strategies, including:
- Quizzes
- Tests
- Projects
These assessments should evaluate both conceptual understanding and problem-solving skills.
Resources for Further Exploration
There are several resources available for students who want to learn more about chemical equation balancing. These resources include:
- Online tutorials
- Videos
- Textbooks
- Online forums
These resources can help students deepen their understanding of the topic and connect with experts in the field.
FAQ: Student Exploration: Balancing Chemical Equations
What is the significance of balancing chemical equations?
Balancing chemical equations ensures that the law of conservation of mass is upheld, guaranteeing that the number of atoms of each element remains constant throughout a chemical reaction.
How does balancing equations aid in predicting reaction outcomes?
Balanced equations provide a roadmap for chemical reactions, allowing scientists to predict the products formed and their relative amounts.
What are the common challenges students face in balancing equations?
Students may encounter difficulties in identifying coefficients, understanding the concept of half-reactions, and applying appropriate balancing methods.
How can students overcome these challenges?
Practice, utilizing step-by-step methods, seeking guidance from teachers or peers, and engaging in interactive learning activities can help students overcome these challenges.