Delving into the realm of lab properties of ionic and covalent compounds, we embark on a scientific expedition that unravels the fascinating characteristics of these two distinct classes of compounds. Their contrasting behaviors in terms of solubility, reactivity, and structure hold the key to understanding their diverse applications in various fields.
As we delve deeper into the topic, we will uncover the intricate crystalline structures of ionic compounds, their remarkable solubility in polar solvents, and their propensity to form ionic bonds. Conversely, we will explore the molecular nature of covalent compounds, their solubility in nonpolar solvents, and the covalent bond formation that governs their chemical behavior.
Physical Properties of Ionic Compounds
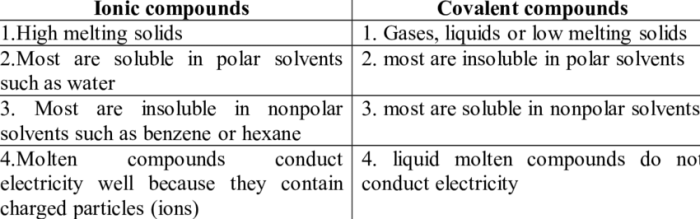
Ionic compounds exhibit distinct physical properties due to their unique crystalline structure and strong electrostatic forces between oppositely charged ions. These compounds are typically crystalline solids at room temperature and possess high melting and boiling points.
Crystalline Structure
Ionic compounds form a regular, repeating arrangement of ions known as a crystal lattice. The positive and negative ions are arranged in an alternating pattern, maximizing the electrostatic attraction between them.
Solubility
Ionic compounds generally exhibit high solubility in polar solvents such as water. The polar solvent molecules interact with the ions, breaking apart the crystal lattice and allowing the ions to dissolve.
Examples
- Sodium chloride (NaCl): White crystalline solid, highly soluble in water
- Potassium chloride (KCl): Colorless crystalline solid, soluble in water
- Calcium fluoride (CaF2): Transparent crystalline solid, sparingly soluble in water
Chemical Properties of Ionic Compounds
Ionic compounds exhibit characteristic chemical properties that result from the strong electrostatic forces between their ions. These compounds tend to react with water and form precipitates in ionic reactions.
Reactivity with Water
Ionic compounds react with water to form hydrated ions. The water molecules surround the ions, forming a hydration sphere that stabilizes them in solution.
Formation of Precipitates, Lab properties of ionic and covalent compounds
When solutions of ionic compounds are mixed, the ions can combine to form insoluble compounds known as precipitates. These precipitates appear as a solid that separates from the solution.
Examples
- Sodium chloride (NaCl) + Silver nitrate (AgNO3) → Silver chloride (AgCl) precipitate + Sodium nitrate (NaNO3)
- Copper sulfate (CuSO4) + Sodium hydroxide (NaOH) → Copper(II) hydroxide (Cu(OH)2) precipitate + Sodium sulfate (Na2SO4)
Physical Properties of Covalent Compounds
Covalent compounds exhibit distinct physical properties due to the covalent bonds that hold their atoms together. These compounds are typically molecular solids, liquids, or gases at room temperature and possess lower melting and boiling points compared to ionic compounds.
Molecular Structure
Covalent compounds are formed by the sharing of electrons between atoms. The atoms are connected by covalent bonds, which are represented by lines in molecular diagrams.
Solubility
Covalent compounds generally exhibit lower solubility in polar solvents such as water. The nonpolar nature of covalent compounds makes them less compatible with polar solvents.
Examples
- Methane (CH4): Colorless gas, insoluble in water
- Ethane (C2H6): Colorless gas, slightly soluble in water
- Ethanol (C2H5OH): Colorless liquid, soluble in water
Chemical Properties of Covalent Compounds
Covalent compounds exhibit characteristic chemical properties that result from the covalent bonds between their atoms. These compounds tend to react slowly and form covalent bonds with other atoms.
Reactivity with Water
Covalent compounds generally do not react with water. The nonpolar nature of covalent compounds makes them less reactive with polar water molecules.
Formation of Covalent Bonds
Covalent compounds form when atoms share electrons to achieve a stable electron configuration. The shared electrons form covalent bonds, which hold the atoms together.
Examples
- Hydrogen (H2): Colorless gas, formed by the covalent bonding of two hydrogen atoms
- Oxygen (O2): Colorless gas, formed by the covalent bonding of two oxygen atoms
- Water (H2O): Colorless liquid, formed by the covalent bonding of two hydrogen atoms and one oxygen atom
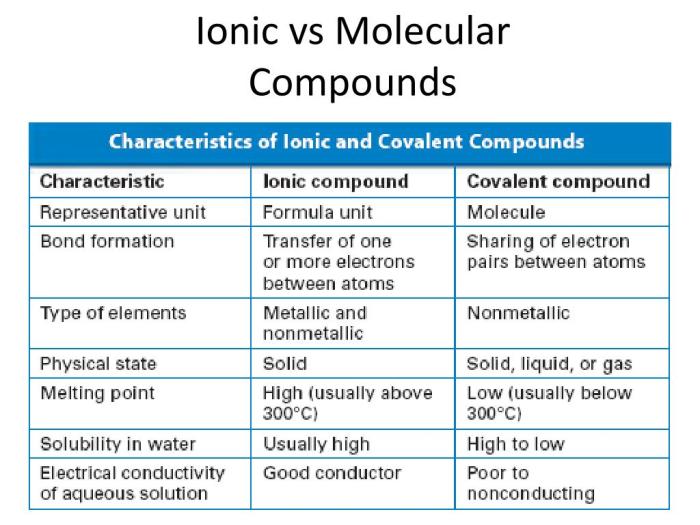
Comparison of Ionic and Covalent Compounds: Lab Properties Of Ionic And Covalent Compounds

| Property | Ionic Compounds | Covalent Compounds |
|---|---|---|
| Structure | Crystalline lattice | Molecular structure |
| Solubility in water | High | Low |
| Reactivity with water | React to form hydrated ions | Do not react |
| Melting and boiling points | High | Low |
| Reactivity | React quickly | React slowly |
FAQ Corner
What is the key difference between ionic and covalent compounds?
Ionic compounds are formed by the transfer of electrons between atoms, resulting in charged ions held together by electrostatic forces. Covalent compounds, on the other hand, are formed by the sharing of electrons between atoms, creating a covalent bond.
Why are ionic compounds generally more soluble in water than covalent compounds?
Ionic compounds dissociate into ions in water, which can interact with the polar water molecules through ion-dipole interactions. Covalent compounds, lacking charged ions, do not readily dissolve in water.
How do the physical properties of ionic and covalent compounds differ?
Ionic compounds tend to have higher melting and boiling points, are typically solids at room temperature, and conduct electricity when dissolved in water. Covalent compounds, in contrast, often have lower melting and boiling points, exist as gases, liquids, or solids at room temperature, and do not conduct electricity in solution.

